In today’s competitive global market, product quality and safety are non-negotiable. Consumers and regulatory bodies demand stringent standards, particularly in industries such as pharmaceuticals, food production, and cosmetics. This is where GMP Compliance comes into play. Good Manufacturing Practices (GMP) ensure that products are consistently produced and controlled according to quality standards, safeguarding the health of consumers.
What is GMP Compliance?
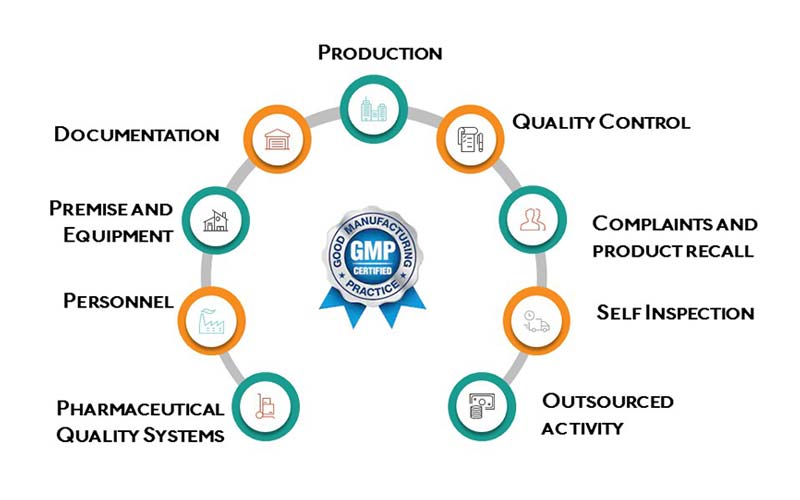
GMP Compliance refers to the adherence to a set of regulations and guidelines that cover all aspects of production, from the sourcing of raw materials to packaging and distribution. It encompasses several critical areas such as:
- Facility and Equipment: Manufacturing plants must maintain clean, controlled environments with calibrated equipment.
- Personnel Training: Employees must be trained regularly in health, safety, and hygiene practices.
- Process Validation: Ensuring that production processes consistently yield products of the highest quality.
- Documentation: Comprehensive records must be maintained for each batch produced, tracking the entire manufacturing process.
Why is GMP Compliance Important?
- Ensures Product Quality and Safety: By adhering to GMP guidelines, companies reduce the risk of contamination, mix-ups, and errors, which can jeopardize product safety.
- Meets Regulatory Requirements: Compliance with GMP standards is mandatory in many countries, especially in highly regulated industries like pharmaceuticals. Failure to meet these standards can result in penalties or even business shutdowns.
- Boosts Consumer Confidence: When consumers see that a company follows GMP guidelines, they feel more secure about the quality of the products they are purchasing.
- Reduces Waste and Costs: Consistently following GMP reduces errors in production, leading to fewer recalls, reworks, and wastage, saving companies time and money.
Key Areas of Focus for GMP Compliance
To achieve GMP Compliance, businesses must pay attention to various key areas:
- Material Control: All materials used in manufacturing must meet defined quality standards and be stored and handled in ways that prevent contamination.
- Sanitation and Hygiene: Ensuring cleanliness in the manufacturing environment and among personnel is vital to prevent product contamination.
- Quality Control: Regular testing of raw materials, intermediates, and final products ensures that everything meets established specifications.
- Packaging and Labeling: Proper labeling and packaging are essential to ensure that consumers receive the correct product with all the necessary information about its use and safety.
The Role of GMP Audits
Regular GMP audits are an essential part of maintaining compliance. These audits help identify potential issues before they become significant problems. Companies that are serious about GMP compliance typically undergo both internal and external audits to continually improve their processes and ensure they are meeting regulatory standards.
Achieving GMP Certification
Achieving GMP Certification is a rigorous process but highly beneficial for companies aiming to elevate their operational standards. It requires an organization to fully implement GMP guidelines, often involving changes in processes, facility upgrades, and extensive documentation. Skydream Digital Innovations (OPC) Pvt. Ltd. offers comprehensive guidance to help businesses navigate this complex process, ensuring they meet all compliance requirements and are well-prepared for inspections.
Conclusion
GMP Compliance is more than just a regulatory requirement—it is a commitment to producing high-quality products that are safe for consumers. By embracing GMP standards, businesses can not only avoid legal pitfalls but also enhance their reputation in the marketplace. With the right guidance, achieving GMP Certification can become a valuable investment in your company’s future.